Order oligos and double-stranding primers
- Dilute stocks to 100uM
- Dilute working stocks of libraries and double-stranding primers to 10uM
- Dilute working stocks of sequencing primers to 3.2uM (6.4uL of stock solution in 193.6uL water)
- Some considerations:
- Oligos should be the maximum length because this will help with PCR cleanup and ligation efficiency
- Make sure you have some spacer sequence around the restriction site. NEB has a list of the length of the spacer sequence required for each restriction enzyme. (8bp is usually a safe bet)
- Order the lowest concentration allowable for the size oligo you want – this will be 50nmole for the 100bp oligo. This will already be more than you’ll need.
- If you don’t mind spending more money you can order special “doped” oligo pools where instead of even concentrations of A/T or A/T/C/G or A/T/C, you get 90%A/2%C/8%G, etc. This allows you to generate a library that is much more likely to produce productive clones.
Double strand the library with modified PCR
- Expected max library size is 108 molecules (limit set by transformation efficiency.) You want to load 10X the expected library size for a single library construction. Therefore, you would like to have 109 molecules for a single transformation.
- 1pmol corresponds to ~1011 molecules
- Use 25pmol of the library to make enough for 2500 transformations
- Total library DNA should be less than ~25pmol per 100uL reaction
Reaction Mix (100uL, 25pmol library)
Use the following reaction mix for each PCR reaction:
- 10 μl 10x Thermo polymerase buffer
- 10 μl 10x dNTPs (10x = 2.5 mM each dNTP)
- 5 μl 10 μM FWD primer
- 5 μl 10 μM REV primer
- 1 μl Polymerase (taq or vent)
- 66.5 μl H2O
- 2.5 μl 10μM library stock
PCR protocol
- 95oC for 2.5 minutes
- Cycle 5 times:
- 55oC (or whatever temperature is appropriate) for 30 seconds (annealing)
- 72oC for 1.5 minutes (elongation)
- 72oC for 10 minutes (final elongation)
- 4oC forever
Perform PCR cleanup on the double-stranded library
- This concentrates the samples and allows for the buffer to be switched to something more appropriate.
- PCR purification columns can handle up to 10ug of DNA
- 100pmol of a 100bp oligo is about 3ug, so multiple 100-ul reactions of 25pmol can be combined into one column
- Expected recovery from a PCR purification reaction is 90% (from the Invitrogen package)
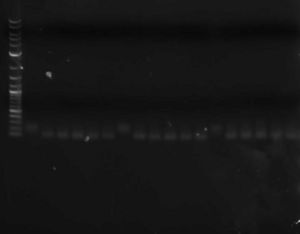
- You can run a sample of the PCR product out on a gel against a sample of the original library to verify that the double-stranding worked (double-stranded DNA should run slightly faster than single-stranded)
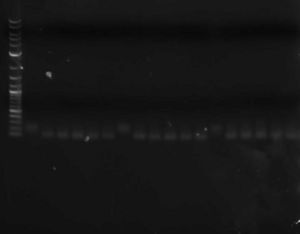 Three libraries ~100bp; on the left is the single-stranded oligo; on the right are double-stranded oligos (different lanes are different primers)
Three libraries ~100bp; on the left is the single-stranded oligo; on the right are double-stranded oligos (different lanes are different primers)